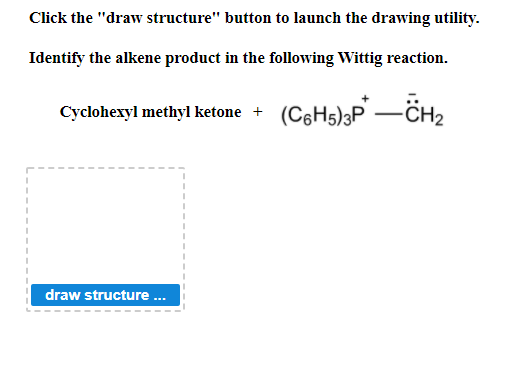
Identify the Alkene Product in the Following Wittig Reaction.
Stabilized ylides tend to form the E isomer of the alkene product whereas unstabilized ylides tend form the Z isomer as the major product. Many are a deep blood red or sometimes grape-juice purple.
This reaction is named for George Wittig who was awarded the Nobel prize for this work in 1979.

. This Reaction is named after its discoverer the German chemist Georg Wittig. The Wittig reaction converts a ketone or aldehyde to a new alkene. The Wittig Reaction allows the preparation of an alkene by the reaction of an aldehyde or ketone with the ylide generated from a phosphonium salt.
Identify the alkene product in the following Wittig reaction. P O alkene triphenylphosphine oxide. Identify the alkene product in the following Wittig reaction.
Rank the following organic compounds in order of increasing basicity. The Ylide The original most widely utilized version of the Wittig reaction makes use of a species known as an ylide pronounced yl-id. What is a limitation of the Wittig reaction.
When it is used for the synthesis of an internal alkene a mixture of E. Identify each of the following structures as either. Therefore alkene I ie 2-methylbut-2-ene is the major product in this reaction.
Identify the alkene product in the following Wittig reaction. Formation of color shows youve made the ylide. On strong heating it decomposes into alkene tert.
If R is an electron withdrawing group then the ylide is stabilized and is not as reactive as when R is alkyl. The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide often called a Wittig reagent to give an alkene and triphenylphosphine oxide. The product alkene and phosphine oxides are normally not colored as is normally true of the phosphonium salt and the carbonyl electrophile.
Identify the alkene product in the following. Thus you can often monitor Wittig reactions by color. Saytzeffs rule implies that in dehydrohalogenation reactions the alkene having a greater number of alkyl groups attached to a doubly bonded carbon atoms is preferably produced.
Previous question Next question. This problem has been solved. The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide often called a Wittig reagent to give an alkene and triphenylphosphine oxide.
95 20 ratings Transcribed image text. Draw the correct products for the given reaction. Disappearance of the color shows that the ylide has reacted and.
Cyclohexyl methyl ketone C6H33P CH33p CH2 draw structure. He was also awarded the 1979 Nobel Prize in Chemistry for this discovery. The Wittig reaction was discovered in 1954 by Georg Wittig for which he was awarded the Nobel Prize in Chemistry in 1979.
Ph Ph A phosphorous ylide R R O ketone or aldehyde solvent R R CH. Identify the alkene that is produced in the following series of reactions. We review their content and use your feedback to keep the quality high.
Wittig reaction is an organic chemical reaction wherein an aldehyde or a ketone is reacted with a Wittig Reagent a triphenyl phosphonium ylide to yield an alkene along with triphenylphosphine oxide. The Wittig reaction was discovered in 1954 by Georg Wittig for which he was awarded the Nobel Prize in Chemistry in 1979. Quaternary ammonium salt of alkyl amines when reacts with silver oxide yield quaternary ammonium hydroxide.
The geometry of the resulting alkene depends on the reactivity of the ylide. Experts are tested by Chegg as specialists in their subject area. Ylides react to give substituted alkenes in a transformation called the Wittig reaction.
The stereoselectivity of the Wittig reaction is due in part to the choice of base and steric effects but is largely controlled by electronic effects. This reaction is known as Hofmann elimination reaction. Thus dehydrohalogenation of the compound yields two alkenes.
The wittig reaction is the best way to make a terminal akene because the other methods form a terminal alkene only as a minor product. A principal advantage of alkene synthesis by the Wittig reaction is that the location of the double bond is absolutely fixed in contrast to the mixtures often produced by alcohol.
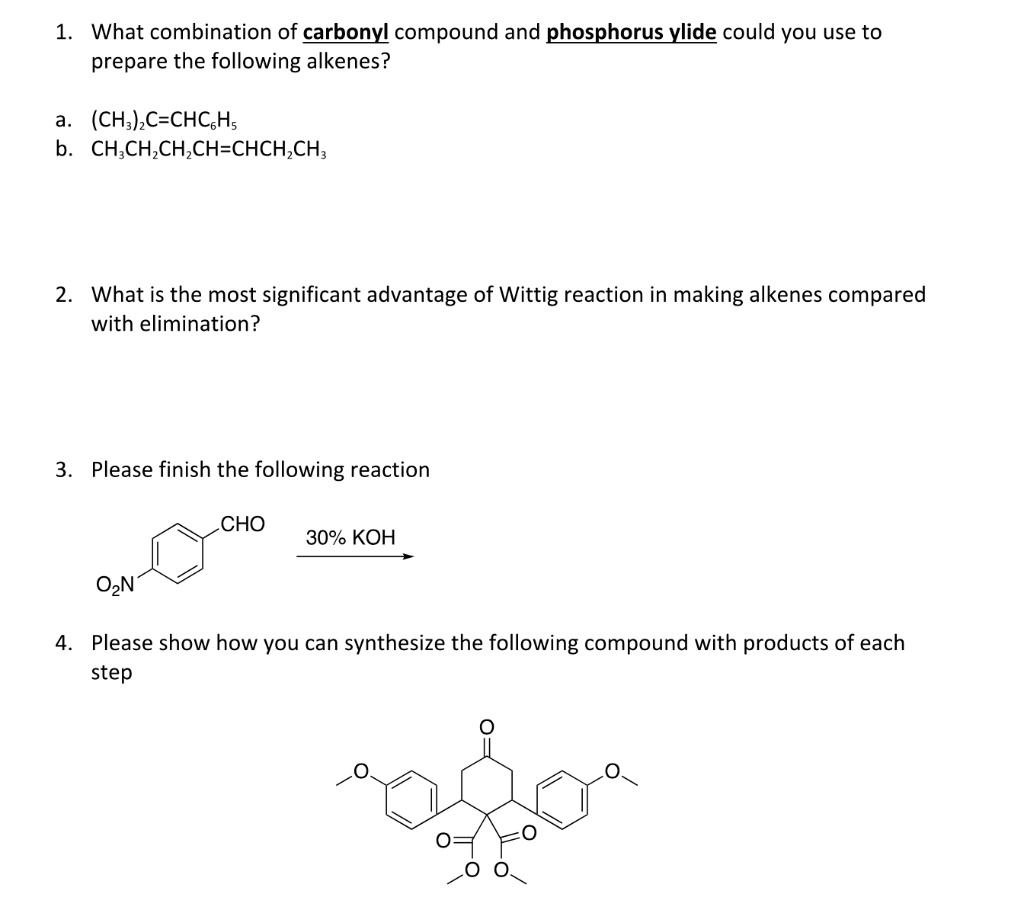
Solved What Combination Of Carbonyl Compound And Phosphorus Ylide Could You Use To Prepare The Following Alkenes Ch C Chcghs B Ch Ch Chzch Chch Ch What Is The Most Significant Advantage Of Wittig Reaction In Making Alkenes Compared
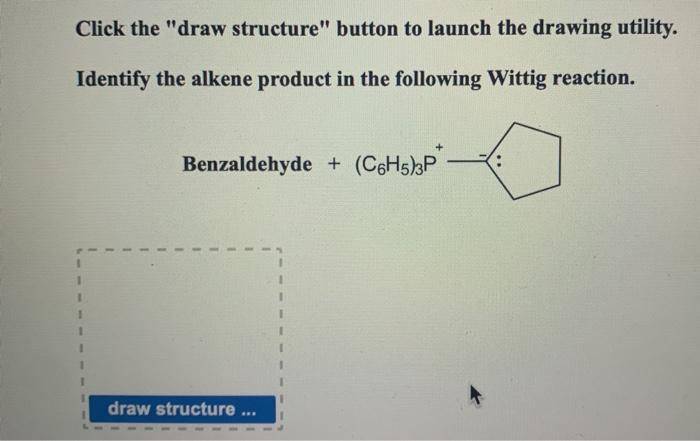
Solved Click The Draw Structure Button To Launch The Chegg Com
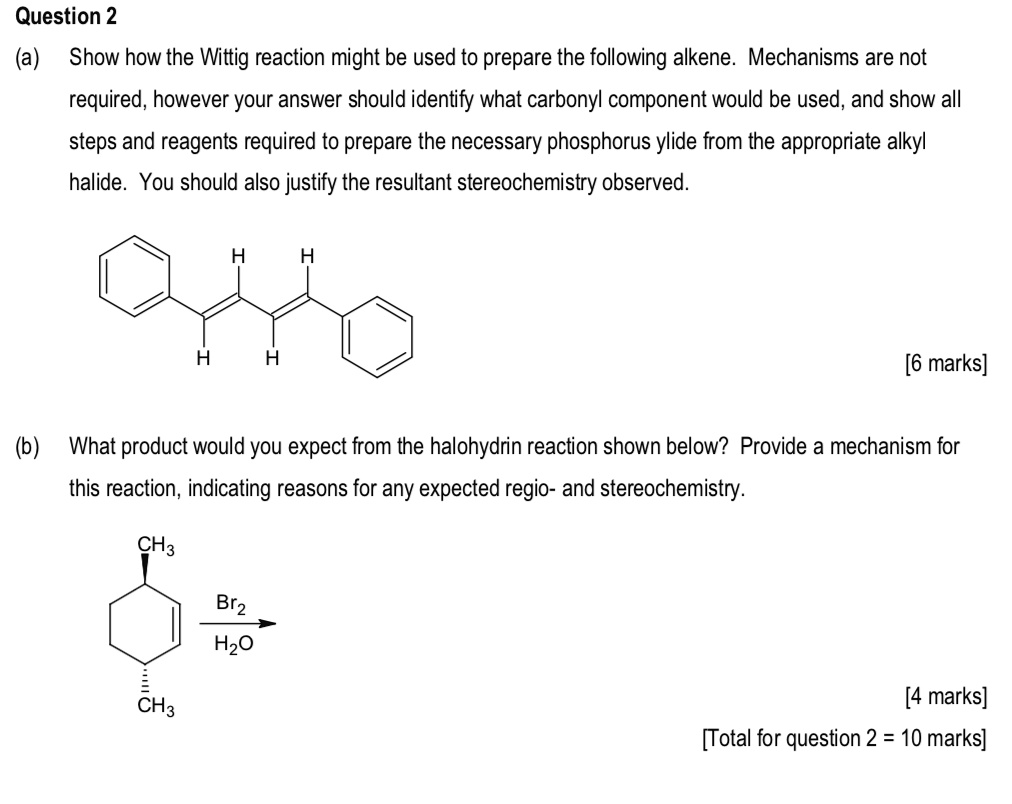
Solved Question 2 A Show How The Wittig Reaction Might Be Used To Prepare The Following Alkene Mechanisms Are Not Required However Your Answer Should Identify What Carbonyl Component Would Be Used And

Alkenes From Aldehydes And Ketones Wittig Reaction Chemistry Libretexts
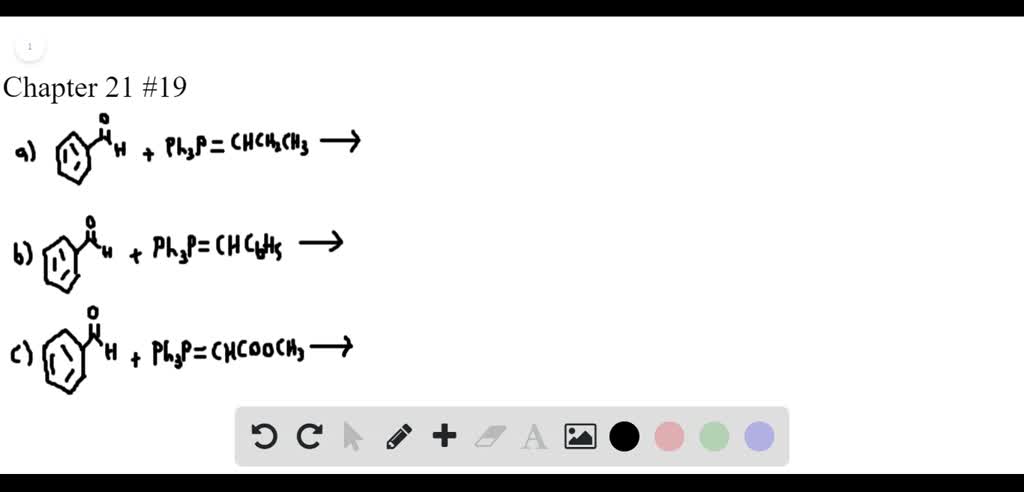
Solved Identify The Alkene Product In Each Of The Following Wittig Reactions A Benzaldehyde Left Left Mathrm C 6 Mathrm H 5 Right 3 Mathrm P Ldots Right B Butanal Left Mathrm C 6 Mathrm H 5 Right 3 Mathrm P
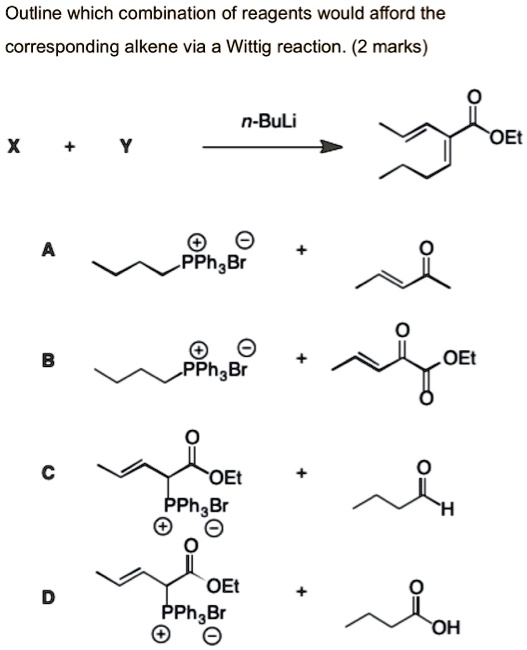
Solved Outline Which Combination Of Reagents Would Afford The Corresponding Alkene Via A Wittig Reaction 2 Marks N Buli Oe X Pphabr Oet Pphabr Oet Pphabr Oet Pphae Br Oh

Alkenes From Aldehydes And Ketones Wittig Reaction Chemistry Libretexts
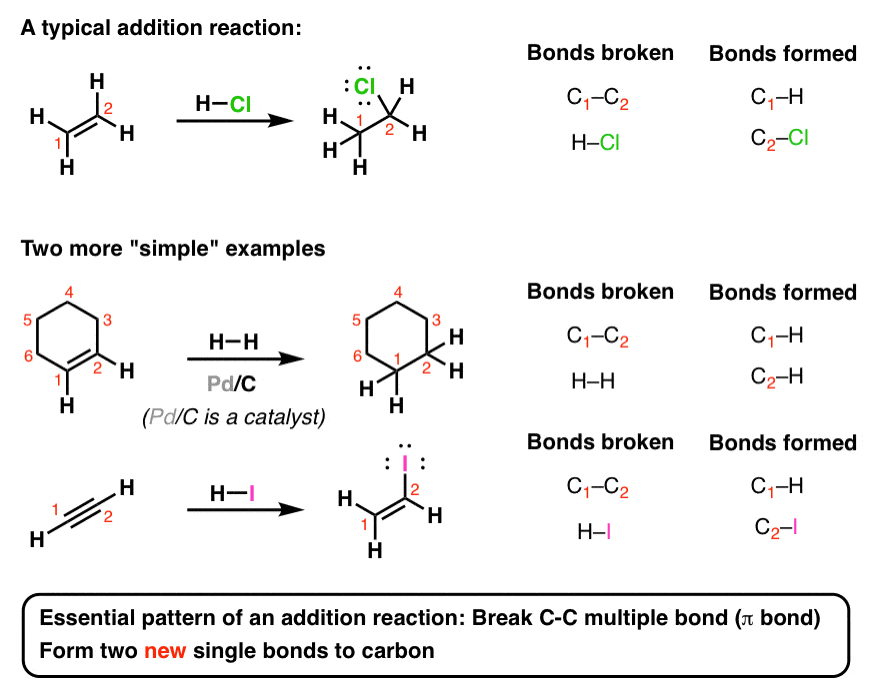
Introduction To Addition Reactions Master Organic Chemistry
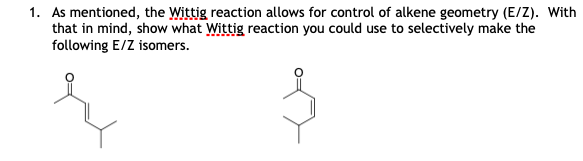
Solved 1 As Mentioned The Wittig Reaction Allows For Chegg Com

Wittig Reaction Mechanism With Reagent Preparation Detailed Explanation

Alkenes From Aldehydes And Ketones Wittig Reaction Chemistry Libretexts
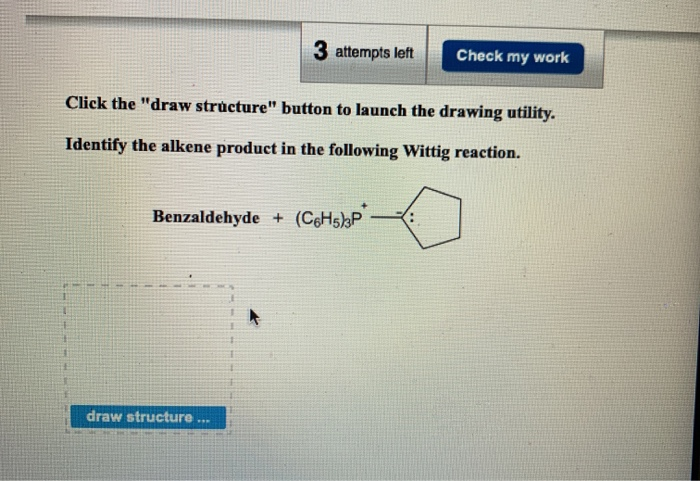
Solved 3 Attempts Left Check My Work Click The Draw Chegg Com
Solved Click The Draw Structure Button To Launch The Chegg Com

Wittig Reaction Examples Wittig Reaction Chemistry Lessons Chemistry

Solved Identify The Alkene Product In The Following Wittig Chegg Com

Wittig Reaction Mechanism With Reagent Preparation Detailed Explanation
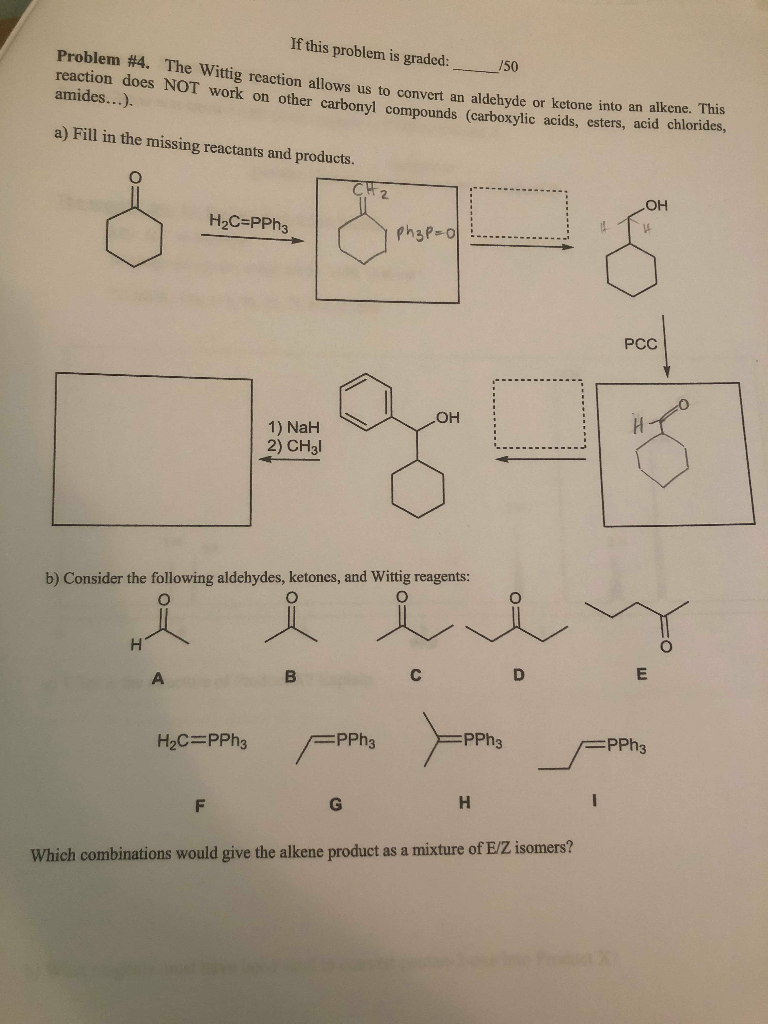
Solved If This Problem Is Graded 150 Problem 4 The Wittig Chegg Com



Comments
Post a Comment